Systemic body odor/halitosis : Do you have bad reactions to drugs/medicines ?
FMO3 is very commonly involved in many oxidation reactions (probably thousands of substrates). Often, a substrate can take an alternative slower route if for some reason the preferred enzyme is saturated (though not trimethylamine, apparently).
In this paper, the researchers looked at the 'clearance' from the blood (detoxification) of the drug Voriconazole. They say that most of the detoxification of voriconazole in humans is done by CYP3A4, CYP2C19, and flavin-containing monooxygenase 3 (FMO3). Apparently in children the clearance rate is faster because they don't use CYP3A4 so much.
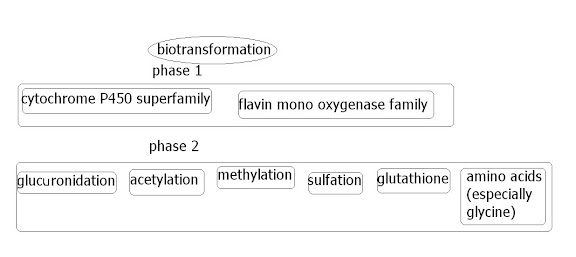 Although researchers learn more about these enzymes, each paper must be taken with some trepidation, in that often on the journey to agreement about facts, they contradict each other. However as time goes by, a picture emerges.
Although researchers learn more about these enzymes, each paper must be taken with some trepidation, in that often on the journey to agreement about facts, they contradict each other. However as time goes by, a picture emerges.Whilst having no FMO3 enzyme does not seem to result in death or visual illness, and those with reduced FMO3 function are expected to live a normal lifespan, the enzyme seems to be involved in thousands of substrate oxidation reactions. So it is likely that most FMO3 research is more likely going to be a consequence to bad reactions to drugs (or uselessness of drugs that these enzymes activate, as well as detoxicate) rather than for trimethylaminuria, which the medical community probably has no interest in. Not unless we can promote or petition for research ourselves.
In Vitro Hepatic Metabolism Explains Higher Clearance of Voriconazole in Children versus Adults: Role of CYP2C19 and FMO3.
Yanni SB, Annaert PP, Augustijns P, Ibrahim JG, Benjamin DK, Thakker DR.
1 The University of North Carolina at Chapel Hill;
Voriconazole is a broad spectrum antifungal agent for treating life threatening fungal infections. Its clearance is approximately three-fold higher in children compared to adults. Voriconazole is cleared predominantly via hepatic metabolism in adults, mainly by CYP3A4, CYP2C19, and flavin-containing monooxygenase 3 (FMO3). In vitro metabolism of voriconazole by liver microsomes prepared from pediatric and adult tissues (n=6/group) mirrored the in vivo clearance differences in children versus adults, and showed that the oxidative metabolism was significantly faster in children compared to adults as indicated by the in vitro half-life (T (1/2)) of 33.8 +/- 15.3 versus 72.6 +/- 23.7 min, respectively. The K(m) for voriconazole metabolism to N-oxide, the major metabolite formed in humans, by liver microsomes from children and adults was similar (11.0 +/- 5.2 muM versus 9.3 +/- 3.6 muM, respectively). In contrast, apparent V(max) was approximately 3-fold higher in children compared to adults (120.5 +/- 99.9 versus 40.0 +/- 13.9 pmol/min/mg). The calculated in vivo clearance from in vitro data was found to be approximately 80% of the observed plasma clearance values in both populations. Metabolism studies in which CYP3A4, CYP2C19, or FMO was selectively inhibited provided evidence that contribution of CYP2C19 and FMO toward voriconazole N-oxidation was much greater in children than in adults, whereas CYP3A4 played a larger role in adults. While expression of CYP2C19 and FMO3 is not significantly different in children versus adults, these enzymes appear to contribute to higher metabolic clearance of voriconazole in children versus adults.
http://www.ncbi.nlm.nih.gov/pubmed/19841059

























0 comments: