Dr John Cashman is a leading (probably THE leading) expert in flavin mono-oxygenase enzyme genetics. He is based at the HBRI in San Diego, that allows anyone to test for trimethylaminuria, both the DNA test (HBRI will do this) and the urine test (presumably sent elsewhere ?). Either test can be done for around $400 each. Although the standard procedure for someone testing for TMAU is to do the urine test first, and if it is negative to not test further, it probably makes sense for the group (and person) to do the DNA test, because the group can then see if this is truly an autosomal recessive problem or not. TMAU is such a new and neglected problem to the medical system that it may turn out their TMAU DNA assumptions are wrong. The main problem being, if not everyone does the DNA test, we don't know what their FMO3 DNA would be. A recent one-off dissertation paper hinted at the notion that TMAU may not be only a recessive problem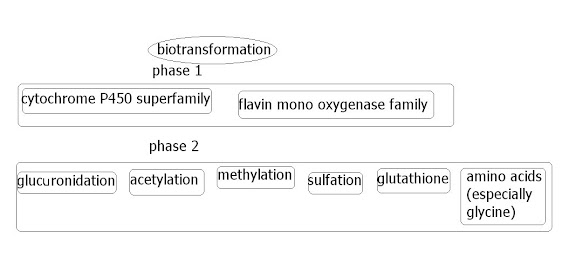
Whilst very few papers are written about FMO enzyme currently, Dr Cashman recently wrote a paper on the FMO family of enzymes. FMO are regarded as part of the 'phase 1' group of xenobiotic-metabolizing/biotransformation enzymes.
FMO3 (the main human isoform) seems to be a very versatile enzyme, neutralizing 1000's of external substrates (for instance through the skin and lungs and from the gut), and internal substrates (neurotransmitters, hormones etc), as well as activating other substrates (such as the active compound in medicines). It deals mostly with soft-nucleophilic heteroatom compounds that include a sulfide, amine, or phospohorous molecule. Since so many sulfides and amines are smelly in various ways, it could be a theory the various smells people suffer from, although currently no smell is accepted as a problem with FMO3 except trimethylamine. Perhaps FMO3 could be suspected as a 'multiple smelly enzyme'.
Unfortunately the paper is not available for free, and so the abstract can only be shownThis review summarizes some recent observations and information related to the role of the flavin-containing monooxygenase (FMO) in preclinical drug development. Flavin-containing monooxygenase is a complimentary enzyme system to the cytochrome P450 (CYP) family of enzymes and oxygenates several soft, highly polarizable nucleophilic heteroatom-containing chemicals and drugs. The products of FMO-mediated metabolism are generally benign and highly polar, readily excreted materials. There may be some advantages in designing drugs that are metabolized in part by FMO and not exclusively by CYP. In this review, I describe the practical aspects for the participation of FMO in drug and chemical metabolism including: i) the study of FMO using in vitro preparations; ii) some observations about metabolism of drugs and chemicals by FMO in vivo; and iii) the consequences of studying FMO-related metabolism in various small animal models. Some of the preclinical research and development areas related to FMO are not fully mature areas and there are certain gaps in our knowledge. However, I include discussion of these areas to stimulate further work and invite further discussion.
http://www.ncbi.nlm.nih.gov/pubmed/19040327
If you go through these links and don't use MEBO on SMILE, MEBO will get ~4%
But ppl forget about these links ....so if u forget then choose MEBO in AMAZON SMILE
MEBO will get 0.5%
but 0.5% of something beats 4% of nothing
Amazon USA
Click here to Amazon USA



























0 comments: